Ozone is an allotrope (a physically or chemically different form of the same substance) of oxygen with the chemical formula O 3 . This formula shows that each molecule of ozone consists of three atoms. By comparison, normal atmospheric oxygen—also known as dioxygen—consists of two atoms per molecule and has the chemical formula O 2 .
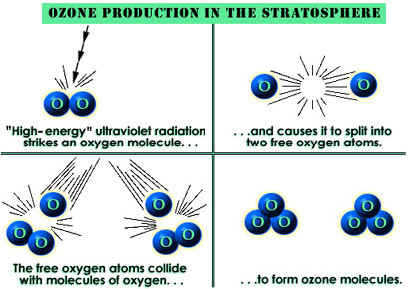
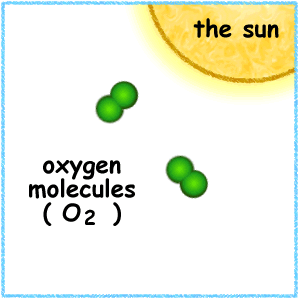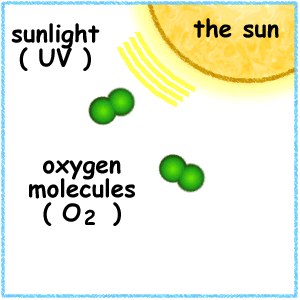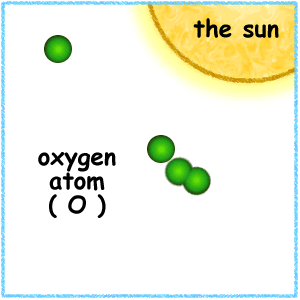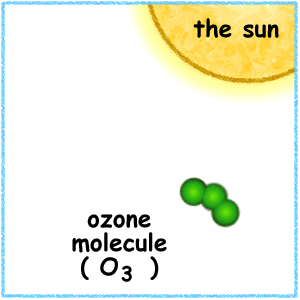
Ozone is a bluish gas with a sharp odor that decomposes readily to produce dioxygen. Its normal boiling point is −112°C (−170°F), and its freezing point is −192°C (−314°F). It is more soluble in water than dioxygen and also much more reactive. Ozone occurs in the lower atmosphere in very low concentrations, but it is present in significantly higher concentrations in the upper atmosphere. The reason for this difference is that energy from the Sun causes the decomposition of oxygen molecules in the upper atmosphere:
O 2 → (solar energy) → 2O
The nascent (single-atom) oxygen formed is very reactive. It may combine with other molecules of dioxygen to form ozone:
O + O 2 → O 3
Words to Know
Allotropes: Forms of a chemical element with different physical and chemical properties.
Chlorofluorocarbons (CFCs): A family of chemical compounds consisting of carbon, fluorine, and chlorine.
Dioxygen: The name sometimes used for ordinary atmospheric oxygen with the chemical formula O 2 .
Electromagnetic radiation: A form of energy carried by waves.
Nascent oxygen: Oxygen that consists of molecules made of a single oxygen atom, O.
Ozone hole: A term invented to describe a region of very low ozone concentration above the Antarctic that appears and disappears with each austral (Southern Hemisphere) summer.
Ozone layer: A region of the stratosphere in which the concentration of ozone is relatively high.
Radiation: Energy transmitted in the form of electromagnetic waves or subatomic particles.
Standard (pollution): The highest level of a harmful substance that can be present without a serious possibility of damaging plant or animal life.
Stratosphere: The region of Earth's atmosphere ranging between about 15 and 50 kilometers (9 and 30 miles) above Earth's surface.
Troposphere: The lowest layer of Earth's atmosphere, ranging to an altitude of about 15 kilometers (9 miles) above Earth's surface.
Ultraviolet radiation: A form of electromagnetic radiation with wavelengths just less than those of visible light (4 to 400 nanometers, or billionths of a meter).
Ozone layer depletion
Most of the ozone in our atmosphere is concentrated in a region of the stratosphere between 15 and 30 kilometers (9 and 18 miles) above Earth's surface. The total amount of ozone in this band is actually relatively small. If it were all transported to Earth's surface, it would form a layer no more than 3 millimeters (about 0.1 inch) thick. Yet stratospheric ozone serves an invaluable function to life on Earth.
Radiation from the Sun that reaches Earth's outer atmosphere consists of a whole range of electromagnetic radiation: cosmic rays, gamma rays, ultraviolet radiation, infrared radiation, and visible light. Various forms of radiation can have both beneficial and harmful effects. Ultraviolet radiation, for example, is known to affect the growth of certain kinds of plants, to cause eye damage in animals, to disrupt the function of DNA (the genetic material in an organism), and to cause skin cancer in humans.
Fortunately for living things on Earth, ozone molecules absorb radiation in the ultraviolet region. Thus, the ozone layer in the stratosphere protects plants and animals on Earth's surface from most of these dangerous effects.
Human effects on the ozone layer. In 1984, scientists reported that the ozone layer above the Antarctic appeared to be thinning. In fact, the amount of ozone dropped to such a low level that the term "hole" was used to describe the condition. The hole was a circular area above the Antarctic in which ozone had virtually disappeared. In succeeding years, that hole reappeared with the onset of each summer season in the Antarctic (September through December).
The potential threat to humans (and other organisms) was obvious. Increased exposure to ultraviolet radiation because of a thinner ozone layer would almost certainly mean higher rates of skin cancer. Other medical problems were also possible.
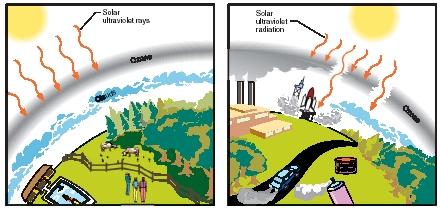
An atmosphere with a relatively intact ozone layer (left) compared to one with an ozone layer damaged by chlorofluorocarbon (CFC) emissions. (Reproduced by permission of
The Gale Group
.)
At first, scientists disagreed as to the cause of the thinning ozone layer. Eventually, however, the evidence seemed to suggest that chemicals produced and made by humans might be causing the destruction of the ozone. In particular, a group of compounds known as the chlorofluorocarbons (CFCs) were suspected. These compounds had become widely popular in the 1970s and 1980s for a number of applications, including as chemicals used in refrigeration, as propellants in aerosol sprays, as blowing agents in the manufacture of plastic foams and insulation, as drycleaning fluids, and as cleaning agents for electronic components.
One reason for the popularity of the CFCs was their stability. They normally do not break down when used on Earth's surface. In the upper atmosphere, however, the situation changes. Evidence suggests that CFCs break down to release chlorine atoms which, in turn, attack and destroy ozone molecules:
CFC → solar energy → Cl atoms
Cl + O 3 → ClO + O 2
This process is especially troublesome because one of the products of the reaction, chlorine monoxide (ClO) reacts with other molecules of the same kind to generate more chlorine atoms:
ClO + ClO → Cl + Cl + O 2
Once CFCs get into the stratosphere and break down, therefore, a continuous supply of chlorine atoms is assured. And those chlorine atoms destroy ozone molecules.
Scientists and nonscientists alike soon became concerned about the role of CFCs in the depletion of stratospheric ozone. A movement then developed to reduce and/or ban the use of these chemicals. In 1987, a conference sponsored by the United Nations Environment Programme resulted in the so-called Montreal Protocol. The Protocol set specific time limits for the phasing out of both the production and use of CFCs. Only three years later, concern had become so great that the Protocol deadlines were actually moved up. One hundred and sixty-five nations signed this agreement. Because of the Protocol, the United States, Australia, and other developed countries have completely phased out the production of CFCs. According to the Protocol, developing nations have until the year 2010 to complete their phase out.
Ozone in the troposphere
Ozone is a classic example of a chemical that is both helpful and harmful. In the stratosphere, of course, it is essential in protecting plants and animals on Earth's surface from damage by ultraviolet radiation. But in the lower regions of the atmosphere, near Earth's surface, the story is very different.
The primary source of ozone on Earth is the internal-combustion engine. Gases released from the tailpipe of a car or truck can be oxidized in the presence of sunlight to produce ozone. Ozone itself has harmful effects on both plants and animals. In humans and other animals, the gas irritates and damages membranes of the respiratory system and eyes. It can also induce asthma. Sensitive people are affected at concentrations that commonly occur on an average city street during rush-hour traffic.
Ozone exposure also brings on substantial damage to both agricultural and wild plants. Its primary effect is to produce a distinctive injury that reduces the area of foliage on which photosynthesis can occur. (Photosynthesis is a complicated process in which plants utilize light energy to form carbohydrates and release oxygen as a by-product.) Most plants are seriously injured by a two- to four-hour exposure to high levels of ozone. But long-term exposures to even low levels of the gas can cause decreases in growth. Large differences among plants exist, with tobacco, spinach, and conifer trees being especially sensitive.
Many nations, states, and cities have now set standards for maximum permissible concentrations of ozone in their air. At the present time in the United States, the standard is 120 ppb (parts per billion). That number had been raised from 80 ppb in 1979 because many urban areas could not meet the lower standard. Areas in which ozone pollution is most severe—such as Los Angeles, California—cannot meet even the higher standard. Measurements of 500 ppb for periods of one hour in Los Angeles are not uncommon.
Long-term problem
Despite a relatively rapid and effective international response to CFC emissions, the recovery of the ozone layer may take up to 50 years or more. This is because these chemicals are very persistent in the environment: CFCs already present will also be around for many decades. Moreover, there will continue to be substantial emissions of CFCs for years after their manufacture, and uses are banned because older CFC-containing equipment and products already in use continue to release these chemicals.
In studies released in late 2000, scientists said they were stunned by findings that up to 70 percent of the ozone layer over the North Pole has been lost and that the ozone hole over the South Pole grew to an expanse larger than North America. According to the National Oceanic and Atmospheric Administration (NOAA), the hole in the ozone layer over the South Pole expanded to a record 17.1 million square miles (44.3 million square kilometers).
Scientists blamed the record ozone holes on two main reasons: extreme cold and the continued use of bromine. Recent very cold winters in the two poles have slowed the recovery of the ozone layer. Cold air slows the dissipation and decay of CFCs, which allows them to destroy ozone faster. Bromine is a chemical cousin to chlorine and is used for some of the same purposes—fire fighting, infection control, and sanitation. NOAA believes bromine is 45 times more damaging to ozone in the atmosphere than chlorine. But bromine has not been regulated as strictly as chlorine because countries could not stand the loss of income if it were regulated more. Some scientists, however, believe governments will be under growing pressure in the coming years to limit the chemical.