
Ozone Creation

One billion years ago, early aquatic organisms called blue-green algae began using energy from the Sun to split molecules of H2O and CO2 and recombine them into organic compounds and molecular oxygen (O2).
This solar energy conversion process is known as photosynthesis.
Some of the photosynthetically created oxygen combined with organic carbon to recreate CO2 molecules. The remaining oxygen accumulated in the atmosphere, touching off a massive ecological disaster with respect to early existing anaerobic organisms. As oxygen in the atmosphere increased, CO2 decreased.

NASA Goddard DAAC

High in the atmosphere, some oxygen (O2) molecules absorbed energy from the Sun's ultraviolet (UV) rays and split to form single oxygen atoms. These atoms combined with remaining oxygen (O2) to form ozone (O3) molecules, which are very effective at absorbing UV rays. The thin layer of ozone that surrounds Earth acts as a shield, protecting the planet from irradiation by UV light.

NASA GSFC
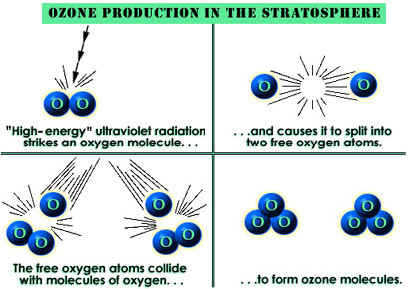
The amount of ozone required to shield Earth from biologically lethal UV radiation, wavelengths from 200 to 300 nanometers (nm), is believed to have been in existence 600 million years ago. At this time, the oxygen level was approximately 10% of its present atmospheric concentration. Prior to this period, life was restricted to the ocean. The presence of ozone enabled organisms to develop and live on the land. Ozone played a significant role in the evolution of life on Earth, and allows life as we presently know it to exist. Ozone is produced naturally in the stratosphere when highly energetic solar radiation strikes molecules of oxygen, O2, and cause the two oxygen atoms to split apart in a process called photolysis.

NASA Goddard DAAC
If a freed atom collides with another O2, it joins up, forming ozone O3. Most of the ozone in the stratosphere is formed over the equatorial belt, where the level of solar radiation is greatest. The circulation in the atmosphere then transports it towards the pole . So, the amount of stratospheric ozone above a location on the Earth varies naturally with latitude, season, and from day-to-day.
Credit University Of Alaska
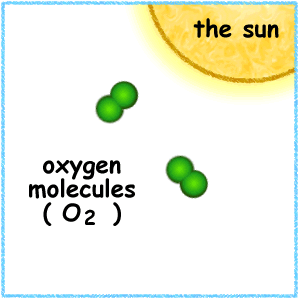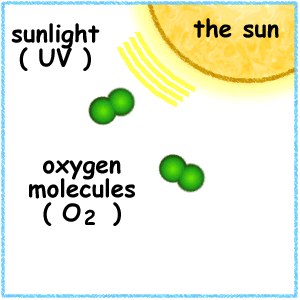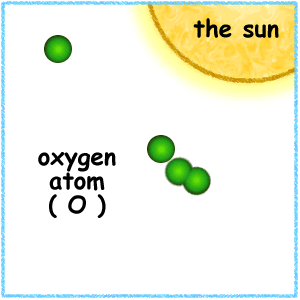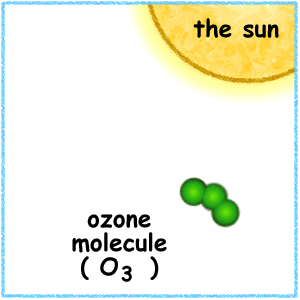
This animation illustrates the formation of ozone. An oxygen molecule (O2) in the stratosphere is broken into 2 oxygen atoms (O + O) by absorbing ultraviolet light energy from the sun. The oxygen atom (O) is now free to react with an oxygen molecule (O2) to create an ozone molecule (O3).
O2 + UV => O + O
O + O2 => O3
Under normal circumstances highest ozone values are found over places such as Canada and Siberia, whilst the lowest values are found around the equator. The ozone layer varies naturally with season. Over Canada is normally about 25% thicker in winter than summer. Weather conditions can also cause considerable daily variations.
Ozone is also naturally broken down in the stratosphere. In an unpolluted atmosphere there is a balance between the amount of ozone being produced and destroyed and so the total concentration remains relatively constant. At different temperatures and pressures (i.e. varying altitudes), there are different production and destruction reaction rates leading to a variation in concentration. The highest ozone concentrations are in the lower stratosphere, between about 18 and 26 km.
Ozone also occurs in very small amounts in the troposphere. It is produced at ground level through a reaction between sunlight and, e.g., gases emitted from cars. As a pollutant it should not be confused with the separate problem of stratospheric ozone depletion.

Ozone creation and depletion process text-
Nenhum comentário:
Postar um comentário